Various gas standards are used when working with medical devices. That is why gas flow analyzers support the conversion vaious gas standards.
What exactly are gas standards?
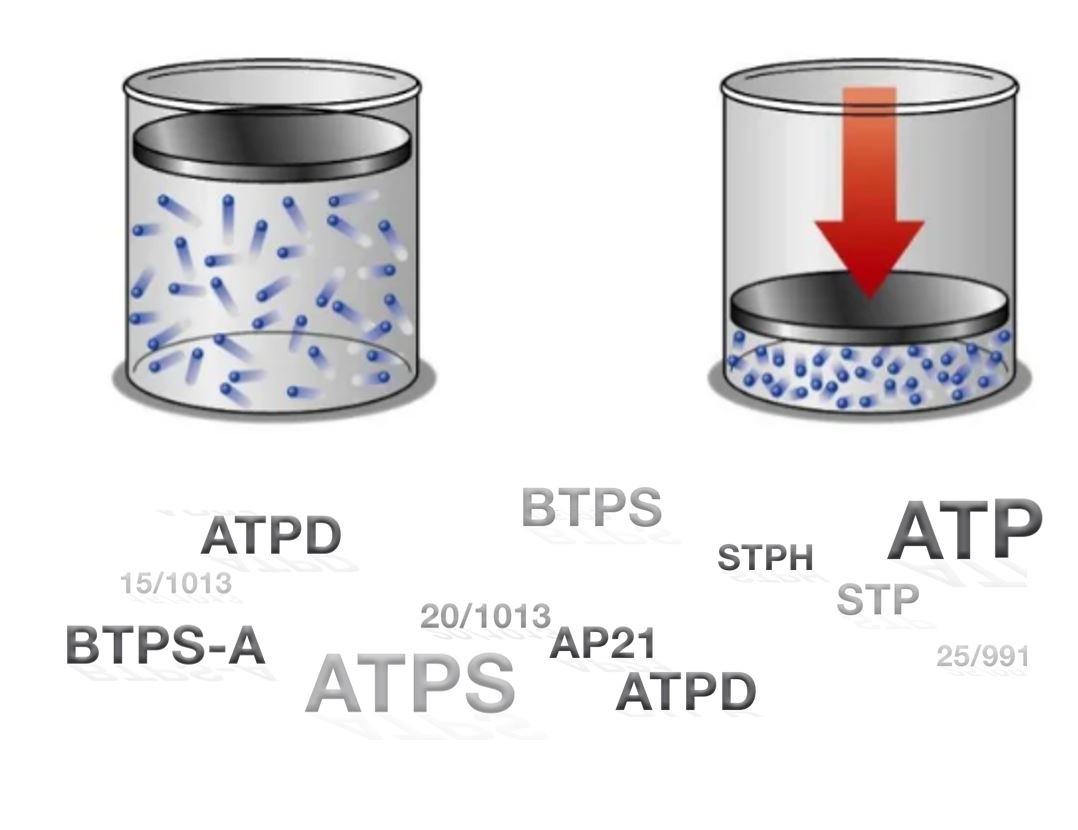
Gas standards describe the gas volume (e.g. Tidal Volume) at specific conditions. These conditions include temperature, pressure, and humidity. Imagine a balloon, filled with air. The air within the balloon has a fixed number of particles, no particles can escape or enter the balloon. If heat up the air in the balloon, it will increase its size. I.e. its volume increases. Hence changing the conditions of the gas, the volume changes while the number of particles stay the same.
Exactly the same is done when we are converting gas standards. But here, this is not only done on volumes but also on volumetric flow. Furthermore, instead of physically changing the conditions, we simply ‘imagine’ changing them. Hence, we basically ask the question: How much flow would we measure if the gas had a different temperature.
Mass flow and volume flow
In some applications, it is beneficial to work with a mass flow instead of a volume flow. In our balloon example, even though the volume changes, the number of particles in the balloon stays constant, hence the mass stays constant. We cannot only express air flow as a flow of volume, but also as a flow of mass. In medical applications, it is common to use a gas standard, where all parameters are fixed. For example, a flow can be expressed in the gas standard STP, where the temperature is fixed to 21.1°C, the pressure to 1013.25mbar, and the relative humidity to 0%. Even though the flow in STP is expressed in volume per time, we get the advantage of mass flow, where the flow stays constant, independent from its true conditions.
Example in medical ventilators
Most ventilators express the measured volume in BTPS (Body Temperature, current Pressure and 100% saturated – humid air) which corresponds to the condition of the exhaled air. Since medical staff want to directly compare inhaled and exhaled air volume, it makes sense to express them in the same gas standard. Otherwise, even though the same number of air particles entered and left the lungs, the expressed volume would be different. Hence, the volume of the inspiratory flow is converted to the conditions of the expiratory flow, such that they can be compared.