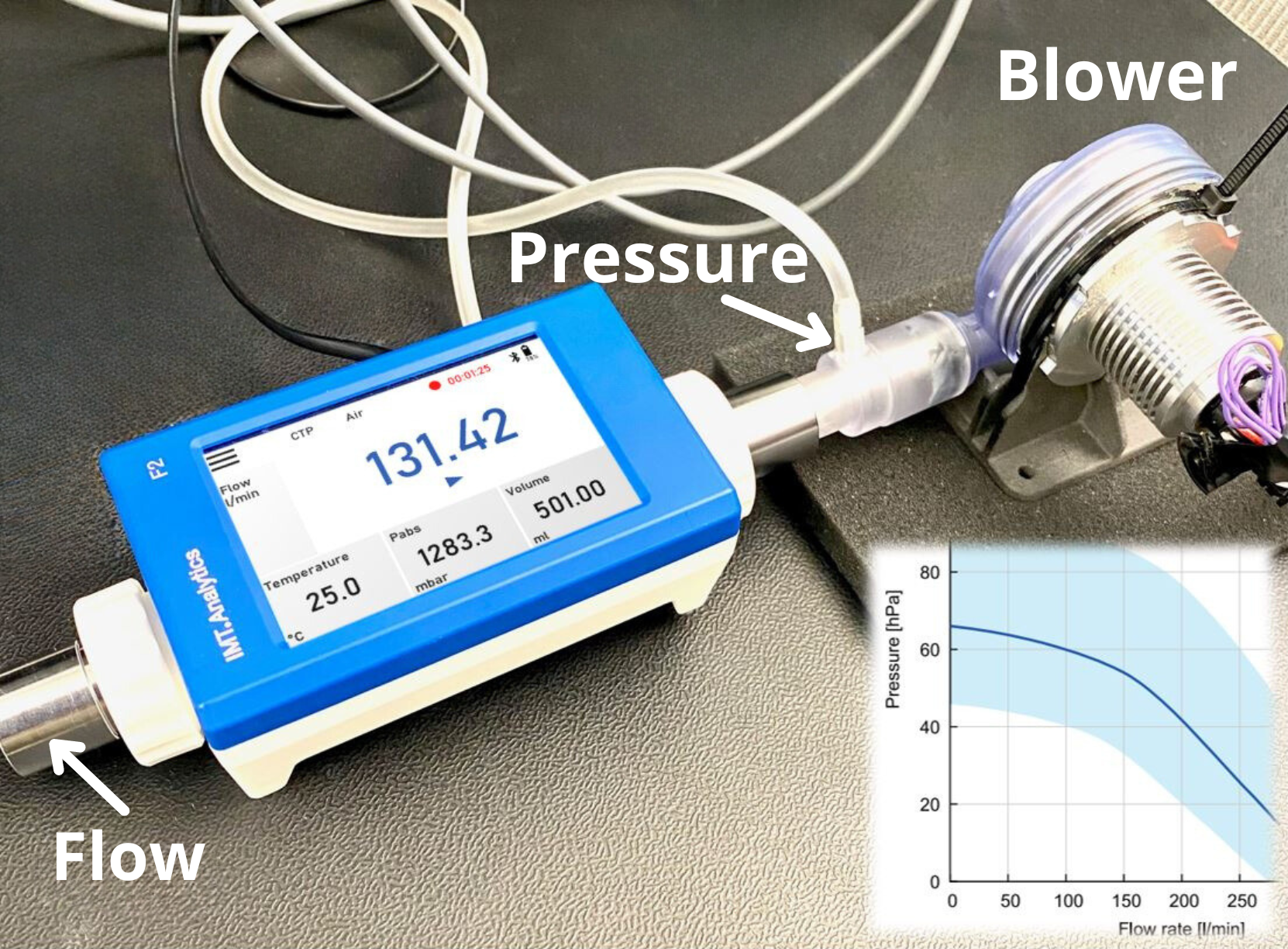
Introduction:
The manufacturing of ventilators, life-saving devices that provide critical respiratory support, requires strict adherence to quality and safety standards. As per FDA 21 CFR Part 820, ventilator manufacturers must establish robust “Production and Process Controls” to ensure consistent product quality. Gas flow analyzers and test lungs emerge as essential tools in this process, playing a crucial role in compliance with regulatory requirements and maintaining optimal performance. In this article, we will explore why gas flow analyzers and test lungs are vital in ventilator manufacturing, aligned with the guidelines outlined in FDA 21 CFR Part 820.
- Calibration of Ventilators:
Accurate calibration of ventilators is essential to ensure precise flow rates and pressure measurements. Gas flow analyzers are instrumental in this process. They provide a means to validate the accuracy and reliability of the ventilator’s flow and pressure measurements. By calibrating ventilators using gas flow analyzers, manufacturers can establish a baseline for accurate performance, enhancing patient safety and maintaining compliance with FDA regulations. - Testing Ventilator Settings and Parameters:
Validating the settings and parameters of ventilators is a critical aspect of production and process controls. Test lungs, in conjunction with gas flow analyzers, enable manufacturers to evaluate and verify the device’s performance under various conditions. Test lungs simulate the respiratory system, allowing for comprehensive testing of tidal volume, respiratory rate, inspiratory/expiratory times, and pressure levels. Gas flow analyzers measure and analyze the flow and pressure delivered by the ventilator during these tests, ensuring that the device operates within the specified parameters. - Independent Measurement and Quality Control:
FDA 21 CFR Part 820 emphasizes the need for robust quality control measures in ventilator manufacturing. Gas flow analyzers serve as independent measurement tools, providing objective and accurate assessments of the ventilator’s performance. By utilizing gas flow analyzers, manufacturers can implement stringent quality control processes that reduce bias and subjective interpretation. These tools enable precise measurements of gas flow and pressure, ensuring consistent and reliable performance in ventilators. - Compliance with Regulatory Standards:
Regulatory compliance is a fundamental requirement for ventilator manufacturers. By incorporating gas flow analyzers and test lungs into their production and process controls, manufacturers can align with FDA 21 CFR Part 820. These tools facilitate rigorous testing, verification, and validation of ventilator performance, ensuring adherence to regulatory standards for accuracy, safety, and reliability. Compliance with these standards is crucial for the marketability and acceptance of ventilators in the healthcare industry.
Conclusion:
Gas flow analyzers and test lungs play a crucial role in the “Production and Process Controls” requirements outlined by FDA 21 CFR Part 820 for ventilator manufacturers. These tools enable accurate calibration, testing of settings and parameters, independent measurement, and adherence to regulatory standards. By incorporating gas flow analyzers and test lungs into their quality control processes, manufacturers can enhance the precision, reliability, and safety of ventilators. Ultimately, this ensures compliance with FDA regulations and instills confidence in the quality and effectiveness of these life-saving devices.