In order for volatile anesthetic agents, which are administered through inhalation and can easily change from liquid to gaseous state, to be used in anesthesia applications, they must be vaporized and administered in specific proportions.
The means used to convert volatile agents from liquid to gaseous state are called vaporizers. The evaporation of the anesthetic agent in a vaporizer depends on several factors, including the boiling point of the agent, the liquid in the vaporizer, the flow velocity, and the heat of the gas passing through the system.
Vaporizers are externally connected devices to the anesthesia system and contain anesthetic liquid agents.
Predominantly, three types of agents are in use: Sevoflurane, Desflurane, and Isoflurane. They are distinguishable by their color and name, and each type of vapor has a different liquid composition. The decision on which agent to use for a specific patient is determined by the anesthesiologist based on the type of surgical procedure.
The vapor, once connected to the anesthesia system, begins to mix with the respiratory system as steam and is transmitted to the patient at the set concentration. Modern anesthesia devices can measure the concentration manually set by the vaporizer through modules and sensors.
This device is highly sensitive and its faulty operation can endanger human life. Therefore, it is crucial to conduct regular maintenance and calibration measurements.
According to the American Society for Testing and Materials (ASTM) standard ASTMF1161-88, anesthetic agent vaporizers are required to be concentration calibrated, meaning a calibrated knob controls the output concentration. Older vaporizers, such as the Copper Kettle and the Vernitrol, lack a single control for selecting the concentration of anesthetic vapor. Where possible, these units should be removed from service.
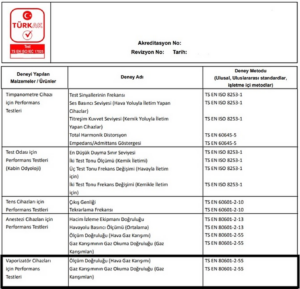
The standard on which the metrological activities of vaporizers are based is ISO 80601-2-55. Additionally, the relevant procedure is updated in the ECRI Biomedical Benchmark IPM, along with the manufacturer’s maintenance standards.
The concentration of the anesthetic gas can be measured with many gas analyzers and sensors available in the market. Current biomedical testing equipment providers offer direct sales and calibration services.
The important point to consider is whether the device you plan to purchase meets the relevant standard. This requires thorough research. For example, a test device with a reading tolerance of ± (0.15 vol% + 5% of the reading) is suitable for calibration measurements of the vaporizer, with a tolerance value of (0.2% volume fraction + 15% adjusted value).
In the photos below, you will see the accredited institutions responsible for test measurements of the Vaporizer and Anesthesia Device in Turkey and the UK.

Biomedical Technician
Medical Device Cal/Test Technician