Abbreviations:
TITCK: Turkish Medicines and Medical Devices Agency
ISO: International Organization for Standardization
IEC: International Electrotechnical Commission
ECRI: Emergency Care Research Institute
The ventilator device is a medical machine located in intensive care units, emergency departments, and mobile health units of healthcare facilities. It delivers a controllable mixture of oxygen and air to the patient’s airways by transmitting the generated gas flow.
This device belongs to the life support group of devices, along with defibrillators, pacemakers, and infusion devices.
The device plays a critical role in connecting the patient to life. Its importance is equally significant for the Clinical Engineering Unit responsible for maintaining the device’s functionality.
Based on its class, the device is usually categorized as a high-risk/major device by its manufacturers and ECRI. Consequently, maintenance, repair, and measurement (calibration) activities for the device are carried out at a high-risk level. For instance, while periodic measurement of an ECG device is typically conducted once a year, the ventilator device requires at least two or more measurement activities per year, depending on its potential use and if the health facility has its own dedicated facilities.
The purpose of maintenance and measurements is to eliminate and minimize potential injuries and deaths caused by the device. Financially speaking, preventive maintenance and metrology activities contribute to utilizing the device for many years to come.
I recommend that the biomedical metrology activity related to this issue ensures that device maintenance is conducted every 3 months. When receiving maintenance services, it is advisable to plan a measurement schedule immediately after maintenance and another measurement schedule during the routine calibration period. By following this approach, the device will be delivered to the user with greater confidence. With operations carried out within this plan and framework, the ventilator device will undergo metrology four times per year.
2 measurements after maintenance/repair
2 periodic biomedical test measurements
Parameters Displayed on the Device and Their Definitions:
It is important to mention the parameters associated with ventilator devices. Depending on the selected mode, the device sets and measures pressure values, volume values, oxygen concentration values using its own sensors, and displays them on the monitor.
Ventilator devices have two main basic modes:
Volume Controlled Ventilation Mode (VC)
Pressure Controlled Ventilation Mode (PC)
Other modes, introduced by different manufacturers using short letters such as BIPAP, PRVC, APRV, SIMV-PCVG, PC-BPAP, VC-MMV, are derived from these two main basic modes and used for different ventilation applications.
Displayed Values:
PEEP: Positive end-expiratory pressure in the lung after exhalation
Ppeak: Peak pressure
Pmean: Mean pressure
Vti: Inspiratory tidal volume
Vte: Expiratory tidal volume
Mv: Minute volume
Ti: Inspiratory time
Te: Expiratory time
I:E: Inspiratory time ratio
O2: Oxygen concentration
Cstat: Static compliance
R: Airway resistance
The device must comply with the ISO 80601-2-12 standard, which specifies requirements for the essential performance and safety of critical care ventilators. It should be manufactured according to this standard and fully meet the basic electrical safety parameters and electromagnetic compatibility standards (IEC 60601-1-1 and IEC 60601-1-2).
Regarding metrological measurements, the same standards apply:
IEC 60601-1-1: Electrical Safety Standard
ISO 80601-2-12: Ventilator Performance Standard
IEC 62353: Electrical Safety Standard (suitable for field personnel)
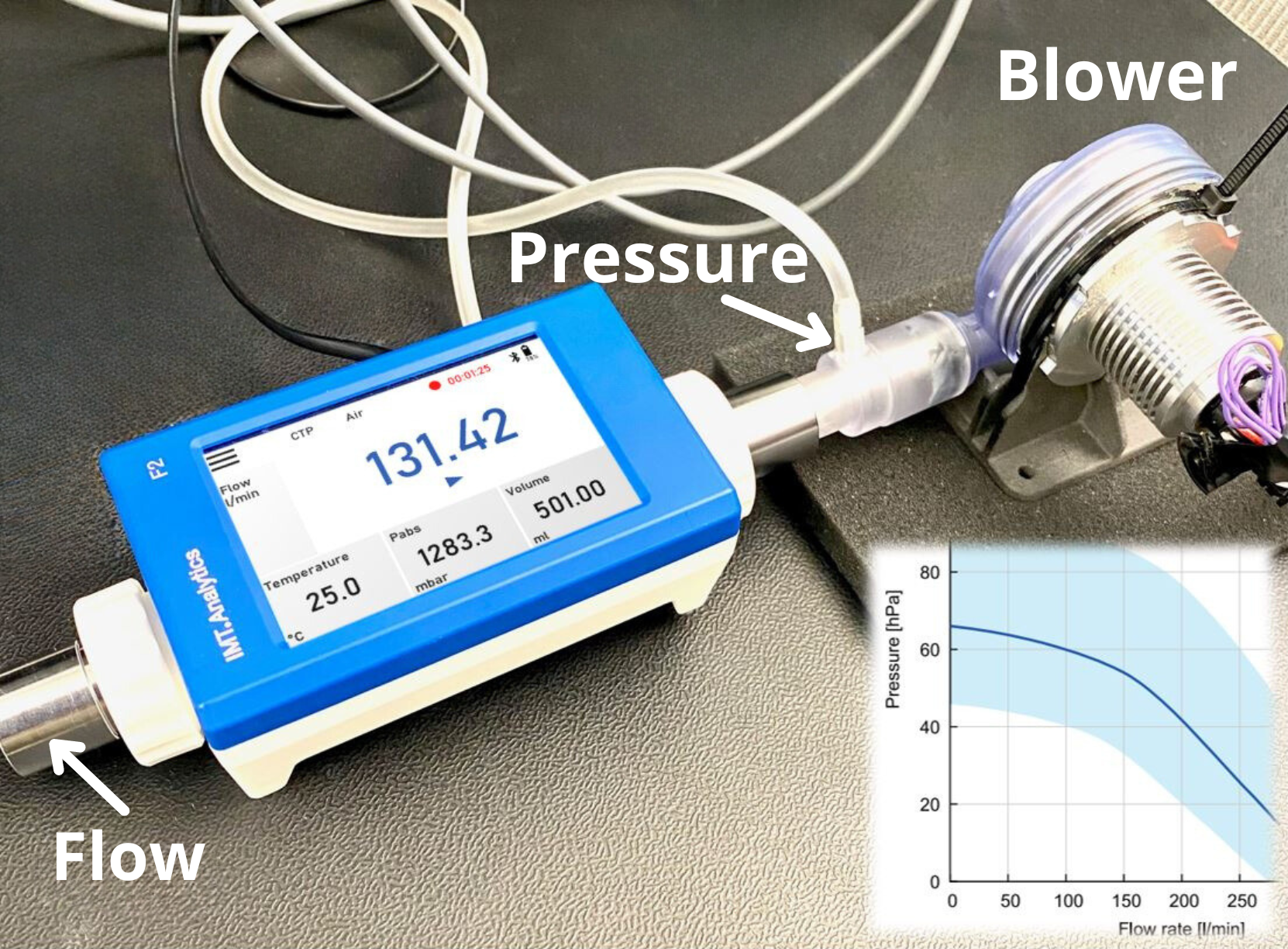
Reference Device & Ventilator Tester
The reference instrument or flow analyzer device has the capacity, high precision, and accuracy to measure all the parameters required for measurement in accordance with ISO 80601-2-12. It is necessary to ensure that the test instrument is within the manufacturer’s specifications and that it undergoes periodic metrology activities, just like the ventilator device subjected to biomedical metrology. If adjustments and measurements of the test instrument can be made, they should be performed by the manufacturer or the official distributor authorized for service and calibration in the country. If this is not possible, it is advisable to have the measurements done by the National Metrology Institute.
It is crucial that the reference device falls within the manufacturer’s specified range to ensure accurate measurements.
My Motto is:
Accurate and Robust Test Tool + Competent Personnel + Correct Method = Clear Result!
Measurement Activity:
Personnel performing metrological activities must have a thorough understanding of the relevant standards, the test equipment used, and the devices being measured. Since ventilator devices come in many brands and models, it may be necessary to seek support from authorized service centers of the respective brands when needed.
Before starting measurements on the instrument, make sure to perform the necessary resets and oxygen sensor calibration in the test instrument. Failure to do so may lead to misleading results.
Another important point for personnel taking measurements on the device is to consider the offset parameter when measuring flow and volume values. Gas standards should be taken into account to accurately compare the displayed measurement values.
For example, let’s consider the city of Buchs, located at an altitude of 450 meters above sea level (960 millibars), with a temperature of 28 degrees Celsius and a relative humidity of 50% on a sunny day. In this example, a gas flow source provides a constant flow of 100 liters per minute.
STP: 100 LPM displayed
ATP: 110.19 LPM displayed
BTPS: 119.04 LPM displayed
As seen in the example, there is a 19% error. Therefore, it is important to know the gas standard in the device being measured before comparing the displayed measurement values. For more information on gas standards, you can visit https://biomedical.blog/gas-standards/.
Weight errors in ventilator devices occur when the flow sensors or cassettes do not provide accurate measurements. The materials in the consumable structure of these devices may lose efficiency due to intensive use and continuous sterilization, resulting in incorrect values or even complete failure.
Ventilator devices have a control mechanism that can perform self-checks, which can identify problems with internal components or connected instruments. It is recommended to carry out this check before subjecting the device to metrology.
Service providers who work with respiratory devices perform maintenance and metrological activities using service and calibration procedures provided by the manufacturers.
It is worth mentioning the ISO 80601-2-12 guidance document mentioned earlier, which includes production and metrological activities. Since ventilator devices are manufactured according to this standard, all technical activities such as medical calibration, reset, offset, and software introductions of flow values with adjustment content must be carried out according to the methods and tests specified in this standard. By following these requirements, service providers and measurement organizations can avoid conflicts and ensure reliable results.
To achieve consensus, service providers may also benefit from participating in “Test, Control, and Calibration Training” conducted by training institutions authorized by TITCK (Turkish Medicines and Medical Devices Agency).
Lastly, it is worth mentioning that in recent years, improvements in anesthesia-related cases indicate that about one-third of reported cases could have been prevented by correctly checking the pre-device itself. This emphasizes the importance of internal checks within the ventilator device.
We can say that:
Competent Users + Right Preventive Care + Right Metrology = Improved Results
Íbrahim PEK
Biomedical Technician
Medical Device Cal/Test Technician