Introduction:
The Centers for Medicare & Medicaid Services (CMS), a federal agency within the U.S. Department of Health and Human Services, plays a critical role in administering the Medicare and Medicaid programs. CMS regulations outline the requirements for healthcare providers participating in these programs, including guidelines for inspecting, maintaining, and testing medical equipment, such as ventilators. In this article, we will explore the importance of adhering to CMS regulations for ventilator maintenance in hospitals and the significance of establishing robust procedures to ensure compliance and quality.
- Regulatory Framework:
CMS regulations mandate that hospitals have procedures in place for inspecting, maintaining, and testing medical equipment, including ventilators. These regulations aim to ensure patient safety, maintain equipment functionality, and prevent adverse events. Hospitals participating in Medicare and Medicaid programs must comply with these requirements to maintain eligibility and provide high-quality care. - Ventilator Inspection:
Regular inspections of ventilators are essential to identify any visible defects, signs of wear and tear, or potential safety concerns. Hospitals are encouraged to establish comprehensive inspection protocols that encompass visual checks for physical damage, loose connections, and proper functioning of control panels and displays. Thorough inspections contribute to early detection of issues, allowing for timely interventions and uninterrupted patient care. - Preventive Maintenance:
CMS regulations emphasize the importance of implementing preventive maintenance programs for ventilators. This involves periodic servicing, cleaning, and calibration activities in accordance with the manufacturer’s guidelines. Preventive maintenance helps prevent malfunctions, ensures optimal device performance, and extends the lifespan of ventilators. Adhering to the recommended maintenance schedules provided by manufacturers is crucial for maintaining compliance. - Testing and Performance Verification:
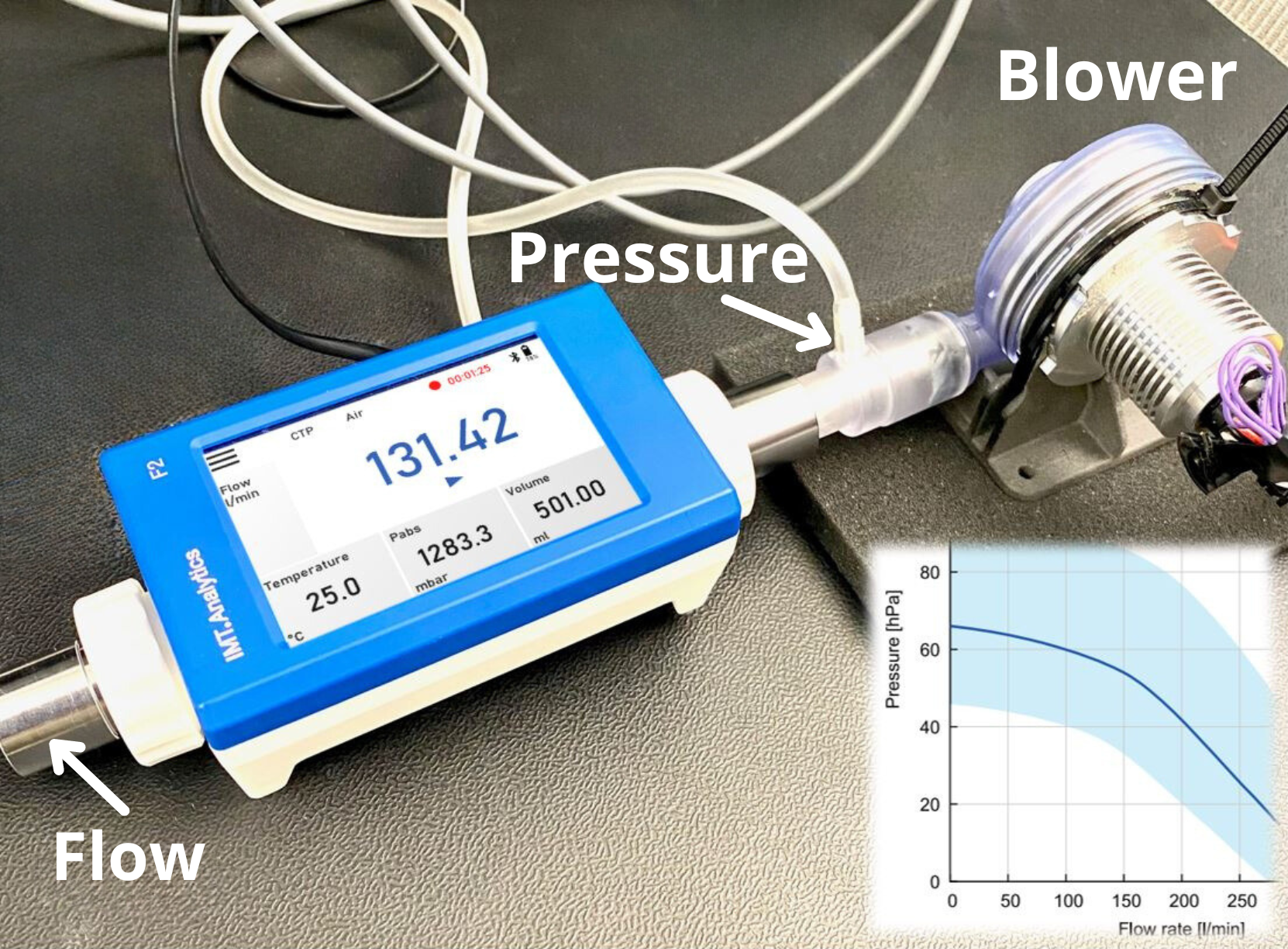
CMS regulations require hospitals to establish procedures for testing and performance verification of ventilators. This includes functional testing to ensure accurate delivery of prescribed parameters, verification of alarm functionality, and assessment of safety features. Regular testing helps identify deviations, malfunctions, or calibration issues, enabling prompt corrective actions and enhancing patient safety. - Documentation and Record-Keeping:
Accurate documentation of ventilator maintenance activities is essential to demonstrate compliance with CMS regulations. Hospitals must maintain records of inspections, preventive maintenance activities, repairs, and testing results. Proper documentation enables traceability, facilitates audits, and serves as evidence of adherence to regulatory requirements. It also assists in tracking the history of each ventilator and identifying patterns or trends related to maintenance and performance. - Hospital Policies and Manufacturer Recommendations:
While CMS regulations provide a framework for ventilator maintenance, hospitals may establish specific policies and procedures tailored to their unique needs. These policies should align with CMS requirements and consider manufacturer recommendations for ventilator maintenance. Manufacturers provide detailed guidelines regarding maintenance schedules, calibration procedures, and recommended spare parts. Hospitals should incorporate these recommendations into their maintenance protocols to ensure optimal device performance.
Conclusion:
Compliance with Centers for Medicare & Medicaid Services (CMS) regulations is crucial for hospitals to uphold quality standards, ensure patient safety, and maintain eligibility for Medicare and Medicaid programs. By establishing comprehensive procedures for ventilator inspection, preventive maintenance, testing, and documentation, hospitals demonstrate their commitment to providing safe and effective care. Adhering to CMS guidelines, along with manufacturer recommendations, enables hospitals to optimize ventilator performance, extend device lifespan, and minimize the risk of adverse events. By prioritizing compliance and quality in ventilator maintenance, hospitals contribute to improved patient outcomes and a culture of excellence in healthcare.