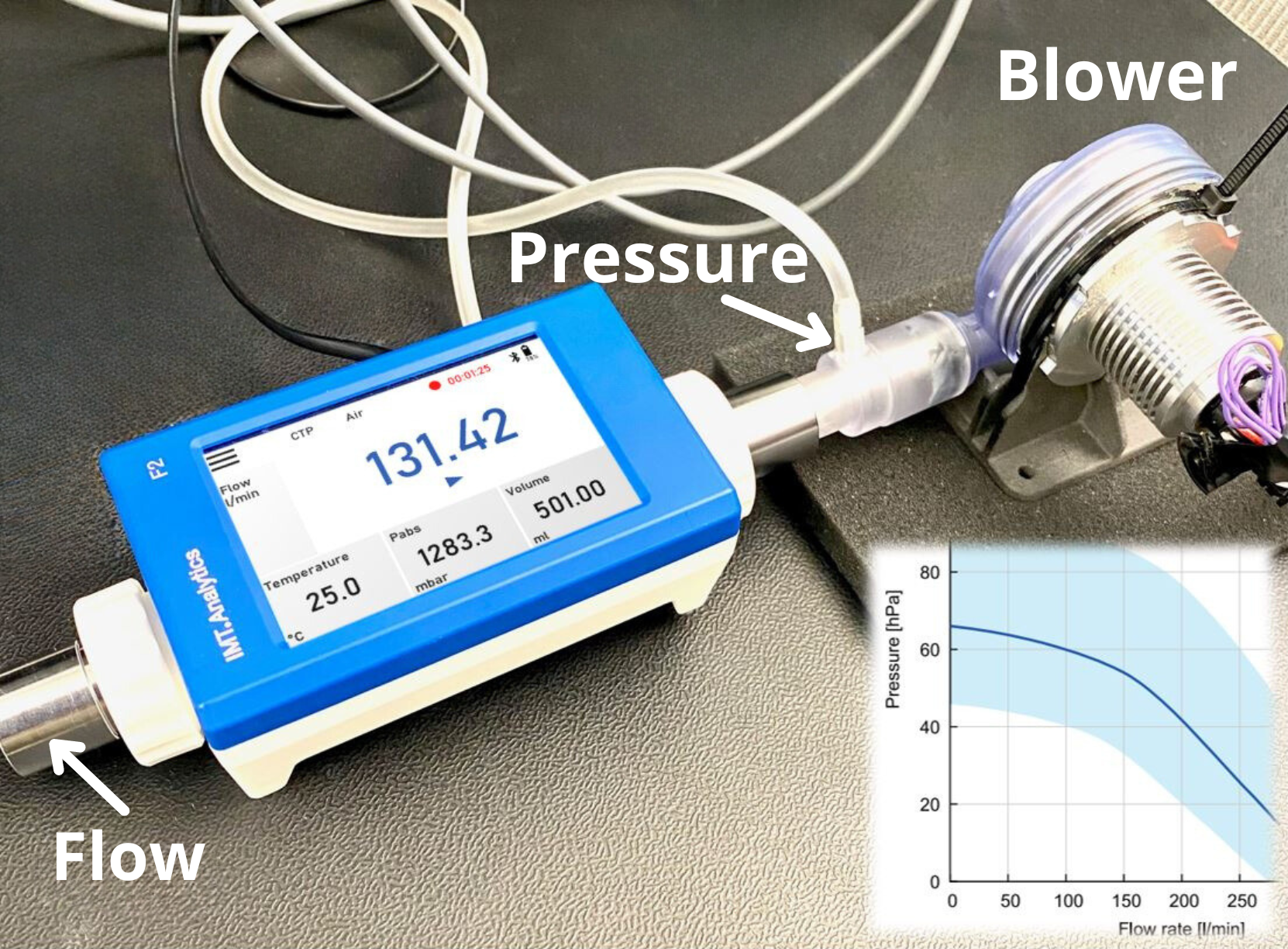
By Frank Schadt, IMT Switzerland
There is enormous potential for AI algorithms in medical technology. Anonymous treatment data can be condensed into therapy recommendations. Device diagnostics can be improved, and lifespan can be increased. Additionally, control and regulation tasks are already being partially taken over by neural networks, decision trees, and similar methods.
However, medical technology also imposes high demands on AI and ML systems. Incorrect decisions can have drastic consequences. The behavior of a neural network may be more difficult to predict than that of a human-developed algorithm. There is an urgent need for regulatory guidelines to ensure the safety of AI systems in medical technology. Therefore, the employees of Swiss IMT Information Management Technology AG from Buchs have been working since the beginning of the year in the IEC PT63450 standards committee – “Artificial Intelligence-enabled Medical Devices – Methods for the Technical Verification and Validation”
The processor performance of embedded devices in medical equipment is often orders of magnitude lower than that of a multimedia PC or production facility. Typical processors in embedded systems are therefore often not powerful enough to handle complex learning tasks. Nevertheless, a large part of popular AI algorithms can be easily integrated into them, if the PC or server takes care of the training. This is because in most cases, training is far more computationally intensive than the subsequent application (inference).
This applies to artificial neural networks (ANNs) as well as to support vector machines, decision trees, and many others. Bayes networks are a counterexample: here, inference is also computationally intensive. This is because conditional probabilities have to be calculated by numerical integration over several variables.