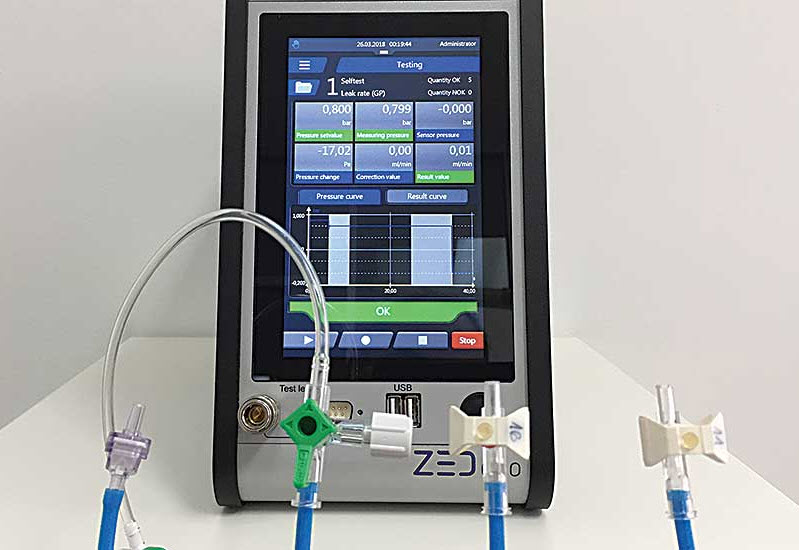
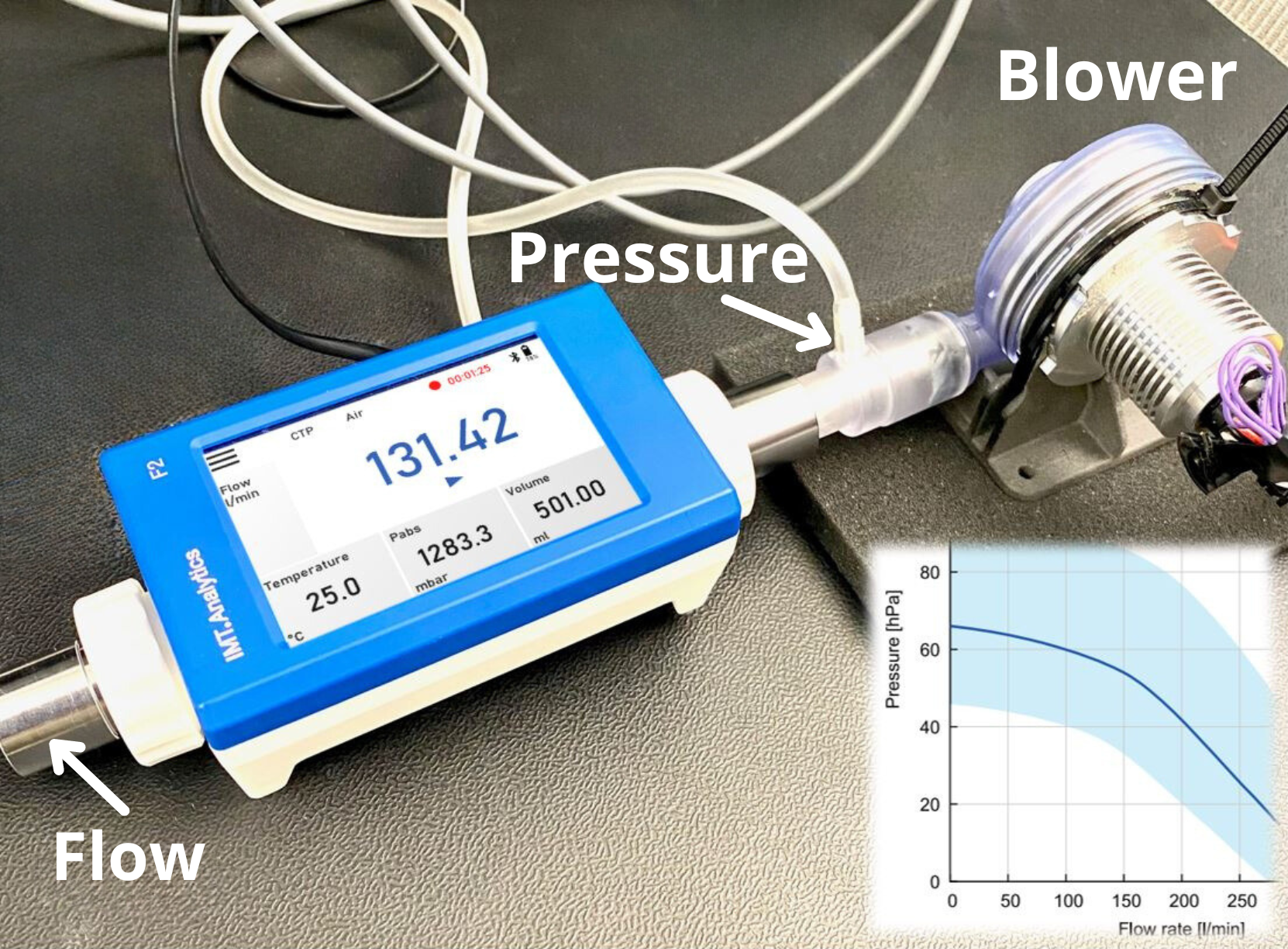
Catheters, pacemakers, ventilators and dialysis filters are vastly different products. But, they all have one thing in common: they must be checked for leaks, whether into, or out of, an assembly. Medical devices often form a barrier between fluids or gases that could create dangerous conditions for patients if they were allowed to mix. Some products are the route for delivery or extraction of fluids. If a leak along the route exists, then the fluids could be delivered to the wrong part of a patient’s body or bodily fluids could gather inside the body. Complete or partial blockage of medical devices could have fatal consequences, as could the failure of a device to deliver the correct dosage. Because many medical devices have fluid management functions that ensure safety and reliability, engineers demand strict parameters for leak testing equipment. “A leaking medical device can cause dire consequences, unlike a product like a waterproof speaker, where a leak just creates a minor inconvenience,” says Jim Burch, sales engineer at ATEQ Corp. “The compressed air supply used for leak testing needs to be of a higher quality than air used to test products such as automotive components or irrigation valves.” “Medical devices have the absolute highest demand on quality, functionality and cleanliness,” adds Juergen Goehner, president of Zeltwanger Leak Testing & Automation LP. “With medical devices, the product is commonly used inside or in direct contact to human bodies. Find more information in the original report in ‘Assemblymag.com’.
Revolutionize Your Medical Device Testing and Calibration with IMT Analytics’ FlowLab software with test sequence
Test Sequences are not just a tool; they are your gateway to unparalleled control and customization. Imagine tailoring testing or calibration procedures to meet the